Biology
>
A-Level
>
Genetics & Health
>
What is th...
What is the sliding filament theory and how is tropomyosin involved?
3 years ago
·
49 Replies
·
15542 views
Dejuan Crooks
49 Answers
The sliding filament theory explains how muscles contract when actin filaments slide over myosin filaments, shortening the sarcomere.
In resting muscle, tropomyosin blocks the binding sites on actin. When a nerve impulse triggers calcium release, the calcium binds to troponin, causing tropomyosin to move and expose the binding sites.
Myosin heads then bind to actin, forming cross-bridges. Using energy from ATP, myosin pulls actin inwards (the power stroke), shortening the muscle. ATP is also needed to detach the myosin head for the next cycle.
When calcium is removed, tropomyosin covers the binding sites again and the muscle relaxes.
The sliding filament theory describes how muscles contract. The two main components are myosin (a thick muscle filament which has characteristic heads protruding from it at regular intervals) and actin (a thin myofilament featuring a myosin binding site).
The myosin binding sites on actin are blocked by tropomyosin. When an action potential arrives at the muscle, this triggers calcium release from the sarcoplasmic reticulum. When the calcium binds to tropomyosin, it pulls it aside leaving the myosin binding sites on the actin free and available.
Myosin heads can now bind to the myosin binding sites on the actin. This is called an actin-myosin cross bridge. A molecule of ATP is hydrolysed which triggers bending of the myosin head, which pulls the actin along. The myosin head then detaches using a molecule of ATP, and binds to the actin at a point further along. The process of 'head bending' is repeated and thus the actin is pulled along by myosin and the muscle contracts.
The analogy of rowing is often used to describe the repeated motion of the myosin head binding, like a boat ploughing through water due to the repeated oar action.
The sliding filament theory is a model of how muscle contraction takes place at a molecular level in skeletal muscle fibres.
There are two types of filaments involved: actin (thin filament) and myosin (thick filament). The actin filament is tightly wrapped in a thinner filament called tropomyosin and also spherical proteins called troponin. The troponin is what tightly binds tropomyosin to the actin filament.
Calcium ions released from the sarcoplasmic reticulum target the troponin proteins and bind to them causing a change in shape. This ultimately loosens the tropomyosin filament thus the actin is less tightly wrapped. In addition to this, myosin binding sites on the actin filament are now exposed.
The sliding filament theory describes the action myosin makes as it "slides" past the actin filament to mediate muscle contraction. The myosin heads bind to the now exposed myosin binding sites on the actin and undergo a series of chemical reactions resulting in the myosin being pulled up each consecutive myosin binding site (much like a rowing team going up a river). Finally, ATP is required to shift the myosin heads back to their starting position to bind to the next myosin binding site.
For muscle contraction, there are actin and myosin filaments. Calcium binds to troponin and changes the shape of one of muscle filament bands. This causes tropomyosin to move and expose myosin binding sites on actin filaments. The actin and myosin filaments form a bridge and slide over each other which causes the muscle fibres to shorten and hence contract.
The sliding filament theory is about how on a cellular and molecular level, a contraction is caused. The sliding filament theory takes place in the sarcomere which is the smallest contractile unit of a muscle in a muscle cell.
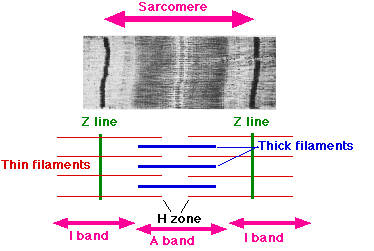 The sarcomere is made up of thin (actin) and thick (myosin) filaments. These are both long filamentous proteins which are found in muscle cells. When a contraction is not being initiated, the molecule tropomyosin is found along the length of the actin filaments covering binding sites for the myosin filaments. This prevents the large majority of the interactions between the proteins. However, when a contraction is initiated, the sarcomere is flooded with Ca2+ ions which induce a confirmational change in the structure of tropomyosin. This causes it to dissociate from actin and allows the myosin filaments to interact and bind to the actin filaments via hockey stick shaped structures called myosin heads. Then, using energy from ATP, the thin and thick filaments will move along each other, causing the sarcomere to become thinner and the muscle to contract.
The sarcomere is made up of thin (actin) and thick (myosin) filaments. These are both long filamentous proteins which are found in muscle cells. When a contraction is not being initiated, the molecule tropomyosin is found along the length of the actin filaments covering binding sites for the myosin filaments. This prevents the large majority of the interactions between the proteins. However, when a contraction is initiated, the sarcomere is flooded with Ca2+ ions which induce a confirmational change in the structure of tropomyosin. This causes it to dissociate from actin and allows the myosin filaments to interact and bind to the actin filaments via hockey stick shaped structures called myosin heads. Then, using energy from ATP, the thin and thick filaments will move along each other, causing the sarcomere to become thinner and the muscle to contract.
 Just to clarify, the colours of actin (thin) and myosin (thick) filaments are reversed between the two pictures so to avoid any confusion ensure to look at the labels provided.
Just to clarify, the colours of actin (thin) and myosin (thick) filaments are reversed between the two pictures so to avoid any confusion ensure to look at the labels provided.
The sliding filament theory is the process of how a muscle contracts. Muscles are made up of many smaller units called myofibrils each containing thousands of contracting units called sarcomeres. These sarcomeres contain myosin (thick) filaments and actin (thin) filaments. On the actin filament are proteins called Troponin and Tropomyosin. When Calcium is released from the sarcoplasmic reticulum, it binds to these proteins and causes them to change shape. This causes tropomyosin to move out of the myosin binding site on the actin filament. This means that the globular head of the myosin filament is free to bind to the actin filament. As it does so, through the use of ATP, it bends and moves the actin filament closer to the centre of the sarcomere, hence causing the myofibril to shorter and the muscle to contract. It then releases itself and binds to another actin filament closest to the sarcomere’s centre and will repeat this process of binding and releasing until the muscle is fully contracted.
The sliding filament theory describes how muscles contract. The two main components are myosin (a thick muscle filament which has characteristic heads protruding from it at regular intervals) and actin (a thin myofilament featuring a myosin binding site).
The myosin binding sites on actin are blocked by tropomyosin. When an action potential arrives at the muscle, this triggers calcium release from the sarcoplasmic reticulum. When the calcium binds to tropomyosin, it pulls it aside leaving the myosin binding sites on the actin free and available.
Myosin heads can now bind to the myosin binding sites on the actin. This is called an actin-myosin cross bridge. A molecule of ATP is hydrolysed which triggers bending of the myosin head, which pulls the actin along. The myosin head then detaches using a molecule of ATP, and binds to the actin at a point further along. The process of 'head bending' is repeated and thus the actin is pulled along by myosin and the muscle contracts.
The analogy of rowing is often used to describe the repeated motion of the myosin head binding, like a boat ploughing through water due to the repeated oar action.
The sliding filament theory is a suggested mechanism of contraction of striated muscles, actin and myosin filaments to be precise, which overlap each other resulting in the shortening of the muscle fibre length. Actin (thin) filaments combined with myosin (thick filaments) conduct cellular movements
The sliding filament theory explain how muscles in the human body contract to produce force.
The steps of this theory are outlined below:
- Muscle Activation: The motor nerve stimulates a motor impulse to pass down a neuron to the neuromuscular junction. It stimulates the sarcoplasmic reticulum to release calcium into muscle cells.
- Muscle Contraction: Calcium floods into the muscle cell and it binds with troponin allowing actin and myosin to bind. The myosin and actin cross-bridges bind and contract using ATP.
- Recharging: ATP is resynthesized which allows actin and myosin to maintain their strong binding state.
- Relaxation: Relaxation takes place when stimulation of the nerve stops. Calcium is then pumped back into the sarcoplasmic reticulum which breaks the link between actin and myosin. Myosin and actin return to their unbound state causing the muscle to relax. Alternatively, relaxation (failure) also occurs when ATP is no longer available.
Hope this helps.
Medical student at UCL looking to help you achieve your academic goals
The sliding filament theory explains how muscles contract once the nervous system receives signals to do. Muscles are made up of contractile units called sarcomeres with thin actin and thick myosin filaments (with the myosin heads binding to the actin-binding site) which then overlap resulting in the shortening of the filament and hence a contraction.
The actin filaments are closely linked with tropomyosin and troponin which are 2 proteins involved in regulation. Once, Ca2+ enters the cell (due to the nervous system) it binds to troponin which then changes shape causing tropomyosin to move. Tropomyosin normally blocks the binding site on actin so movement causes the binding site to be open and therefore allowing myosin to bind.
I'm available for 1:1 private online tuition!
Click here to view my profile and arrange a free introduction.The sliding filament theory describes how muscles contract by detailing the interaction between actin and myosin filaments within a sarcomere, the basic unit of muscle fibers. During contraction, the thin actin filaments slide past the thick myosin filaments, shortening the sarcomere and leading to the overall contraction of the muscle.
Tropomyosin plays a crucial regulatory role in this process. Tropomyosin is a long, fibrous protein that winds around the actin filaments. In a resting muscle, tropomyosin covers the myosin-binding sites on the actin filaments, effectively preventing the myosin heads from attaching to actin and forming cross-bridges. This ensures that the muscle remains in a relaxed state and does not contract unless a specific signal is received.
When the muscle is stimulated to contract, tropomyosin shifts its position on the actin filament, uncovering the myosin-binding sites. This exposes these sites so that the myosin heads can attach to actin and initiate the contraction cycle. By regulating access to the binding sites, tropomyosin ensures that cross-bridge formation only occurs when it is appropriate.
Once the contraction is complete, tropomyosin returns to its original position, re-covering the binding sites on actin and preventing further interaction with myosin. This allows the muscle to relax and prevents unwanted or continuous contraction. In this way, tropomyosin functions as a gatekeeper, controlling the initiation and cessation of the contraction cycle by regulating the accessibility of actin’s binding sites for myosin.
The sliding filament theory is a widely accepted explanation of how muscles contract to produce movement. It describes the process of muscle contraction based on the sliding of actin (thin filaments) and myosin (thick filaments) past one another. This sliding shortens the sarcomere, the functional unit of a muscle, leading to overall muscle contraction.
Key Steps in the Sliding Filament Theory:
- Signal Initiation: A nerve impulse triggers the release of calcium ions (Ca²⁺) from the sarcoplasmic reticulum into the muscle cell's cytoplasm.
- Calcium Binding: Ca²⁺ binds to troponin, a regulatory protein on the actin filament.
- Tropomyosin Shift: In the resting state, tropomyosin blocks the binding sites for myosin on actin filaments. When calcium binds to troponin, it causes a conformational change in the troponin-tropomyosin complex, moving tropomyosin away from the binding sites.
- Cross-Bridge Formation: Myosin heads attach to the exposed binding sites on actin, forming a cross-bridge.
- Power Stroke: Using energy from ATP, the myosin heads pivot, pulling the actin filaments toward the center of the sarcomere.
- Detachment and Resetting: Another ATP molecule binds to the myosin head, causing it to detach from actin and reset to its original position.
- Repetition: This cycle repeats as long as calcium and ATP are available, allowing filaments to slide further and the muscle to contract.
Role of Tropomyosin:
Tropomyosin is a regulatory protein that wraps around the actin filament. In its resting state:
- Blocks myosin-binding sites: Tropomyosin prevents myosin from binding to actin, ensuring that muscle contraction does not occur without proper signaling.
- Enables regulation by calcium and troponin: Tropomyosin's position is controlled by troponin, which responds to calcium levels. When calcium binds to troponin, tropomyosin shifts to uncover the binding sites, facilitating the contraction process.
The sliding filament theory explains how muscles contract to bring about a force. Thin actin filaments and thick myosin filaments within a muscle fibre slide past each other to shorten the length of the muscle without changing the length of the overall muscle filament. Tropomyosin is a protein which prevents myosin binding to actin when muscle is relaxed. It does this by blocking the myosin binding sites on actin. Actin has another regulatory protein called troponin which has a similar function to tropomyosin. When calcium binds to troponin, tropomyosin shifts position to open the myosin binding site. This allows the formation of a cross-bridge between actin and myosin which is key for contraction. Using energy from ATP hydrolysis, myosin pulls the actin filament inwards in a move called a “power stroke”. When a new ATP binds to myosin, it releases actin and the cycle repeats itself to shorten the length of the muscle.
Sliding filament theory or power stroke explains how actin and myosin filaments slide over each other to produce a muscle contraction in skeletal muscle. Once a nerve impulse/action potential travels down the the T-tubule deep into a muscle fibre, it causes the release of calcium ions from sarcoplasmic reticulum. The calcium ions bind to troponin (a protein molecule attached to tropomyosin) which causes a conformational change and pulls the tropomyosin filaments aside. This exposes the myosin head binding site on actin filaments, The myosin head is now able to bind onto actin filament forming a cross bridge pulling actin filament. ATP molecule attaches to myosin head, hydrolysis of which causes the myosin head to detach and come to original shape before binding to the next myosin head binding site.
Qualified Medic. Advancing research and education for 3+ years
5 reviews
The sliding filament theory explains how muscle contraction occurs at the molecular level, particularly in skeletal muscle. It involves the interaction between two types of filaments: actin (thin filaments) and myosin (thick filaments). These filaments slide past each other to shorten the muscle fibre and generate force.
In a relaxed muscle, the actin filaments are partially covered by tropomyosin, a regulatory protein that blocks the binding sites on actin for the myosin heads. Tropomyosin is anchored to the actin filament by another protein called troponin. The troponin-tropomyosin complex prevents myosin from binding to actin at rest, thus inhibiting muscle contraction.
When a muscle is stimulated by an action potential from a motor neuron, calcium ions (Ca²⁺) are released from the sarcoplasmic reticulum into the cytoplasm of the muscle cell.
The rise in intracellular calcium concentration triggers the binding of calcium to troponin, a component of the troponin-tropomyosin complex. This binding causes a conformational change in the troponin molecule.
The conformational change in troponin pulls the tropomyosin away from the binding sites on actin, exposing them to the myosin heads.
The myosin heads, which are in a "cocked" position due to the hydrolysis of ATP into ADP and inorganic phosphate, bind to the exposed binding sites on actin, forming a cross-bridge. This process is facilitated by the energy from ATP hydrolysis.
Once the myosin head is attached to actin, the release of ADP and inorganic phosphate causes the myosin head to pivot, pulling the actin filament towards the centre of the sarcomere. This is known as the power stroke, and it results in the sliding of actin over myosin, shortening the muscle fibre and producing muscle contraction.
A new ATP molecule binds to the myosin head, causing it to detach from the actin filament. The ATP is hydrolysed, re-cocking the myosin head to its original position, ready for another cycle of binding and pulling. This cycle repeats as long as calcium remains elevated in the cytoplasm and ATP is available.
When the action potential ceases, calcium ions are actively pumped back into the sarcoplasmic reticulum. As calcium levels drop, calcium dissociates from troponin, allowing tropomyosin to move back and block the actin-binding sites, thus preventing further cross-bridge formation and causing muscle relaxation.
Tropomyosin, as part of the troponin-tropomyosin complex, plays a crucial role in regulating muscle contraction by controlling the access of myosin to actin. Without the conformational changes triggered by calcium binding to troponin, tropomyosin remains in a position that blocks myosin from attaching to actin. This dynamic regulation ensures that contraction only occurs when appropriate signals are present.
An application of knowledge to medicine:
The use of drugs like calcium channel blockers or muscle relaxants (such as verapamil and diltiazem), are commonly used to manage conditions like hypertension and angina. These medications inhibit the influx of calcium into cardiac and smooth muscle cells by blocking the L-type calcium channels on the cell membrane. In the context of muscle contraction, the reduction in calcium influx inhibits the excitation-contraction coupling process in cardiac muscle. This leads to decreased heart rate, contractility, and vascular resistance, providing relief in conditions like angina or arrhythmias that affect calcium release or muscle contraction requires a detailed understanding of excitation-contraction.
I'm available for 1:1 private online tuition!
Click here to view my profile and arrange a free introduction.Think you can help?

Need an A-Level Biology tutor?
Get started with a free online introductions with an experienced and qualified online tutor on Sherpa.
Find an A-Level Biology Tutor