Physics
>
GCSE
>
Measuring and Detecting Radioactivity
>
How do fir...
How do fire alarms use alpha radiation?
4 years ago
·
1 Reply
·
4426 views
Jena Wuckert
1 Answer
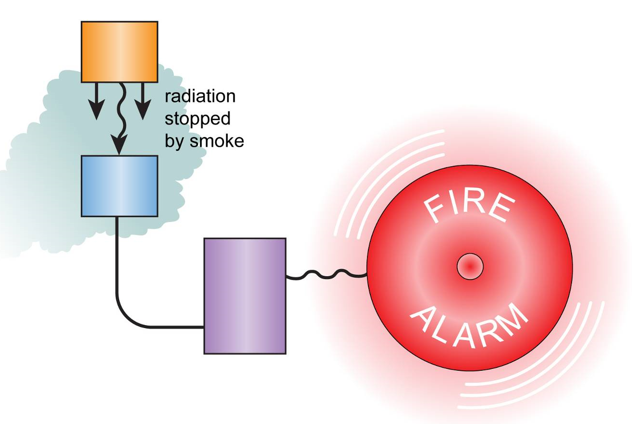
Fire alarms (more specifically smoke detectors) have a very small mass of a substance known as a radio-isotope in them.
This radio-isotope is simply an element that emits nuclear radiation in the form of alpha particles.
Next to this element is an alpha particle detector. The detector is very close to the element emitting the radiation which means it should always be able to detect the radiation it is emitting.
Alpha particles are very poor at penetrating substances - even a few centimetres of air will stop them.
During a fire smoke is usually produced which contains large particles of carbon - the black colour in smoke.
If these smoke particles enter the smoke detector, they are big enough to block the alpha particles from getting to the alpha particle detector in the alarm. When this happens the alarm will go off.
Think you can help?

Need a GCSE Physics tutor?
Get started with a free online introductions with an experienced and qualified online tutor on Sherpa.
Find a GCSE Physics Tutor