Chemistry
>
KS3
>
Classifying Materials
>
How would ...
How would you describe the typical density of a gas?
4 years ago
·
4 Replies
·
3689 views
Nicolette Mraz
4 Answers
UCL Medical student with 4 years of tutoring experience
5 reviews
Typically the density of gas is very low. There are very few particles per unit of volume because particles are randomly arrnaged and spaced out. Remeber density refers the number of particles per unit volume.
I'm available for 1:1 private online tuition!
Click here to view my profile and arrange a free introduction.A very experienced teacher with a natural empathy for students
Of all the common states of matter, gases have the lowest densities because their atoms or molecules are furthest apart from one another with typically no or hardly any interaction. Typically then you could expect a metre cubed of gas to weigh about 1 kilogram i.e the density is about 1kg/m^3.
I'm available for 1:1 private online tuition!
Click here to view my profile and arrange a free introduction.Gases have very low density compared to liquids and solids. Let’s look at why.
Density is a measure of how packed the particles are in any given material/substance/thing . Density is defined as mass( mass is measure of how many particles there are) per unit volume(a unit volume means one unit of volume such as 1cm3, 1m3, 1 dm3, 1 mm3 etc). In a gas the particles are spread apart the most, compared to liquids and solids. Therefore, in a given unit of volume there will be lesser number of particles in a gas compared to the number particles in the same volume of liquid and solid. This makes the gases less dense. Let’s try to understand in the context of the density formula as well.
The formular for working out the density of a substance: 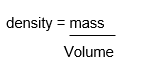
For example, if we consider taking 1cm3 of a gas, liquid and a solid, the mass (the top number) is lowest for the gas. This is because the particles are far apart in a gas. If the particles are spread apart a lot then there will fewer number of particles making the mass very low of the gas. In this example we know that we are taking 1cm3 of each, so the bottom number remains the same. What changes is the top number for the three states. Smaller the top number, smaller the density value, which means less dense the gas is.
This is why you can lift a big inflated beach ball very easily than a small brick, it's the same reason why a beach ball would float and the small brick would sink if you throw them both to the water.
A gas is typically thin and spread out, with particles far apart. Its density is much lower than liquid or solids, eaning it takes up a lot of space.
Think you can help?

Need a KS3 Chemistry tutor?
Get started with a free online introductions with an experienced and qualified online tutor on Sherpa.
Find a KS3 Chemistry Tutor